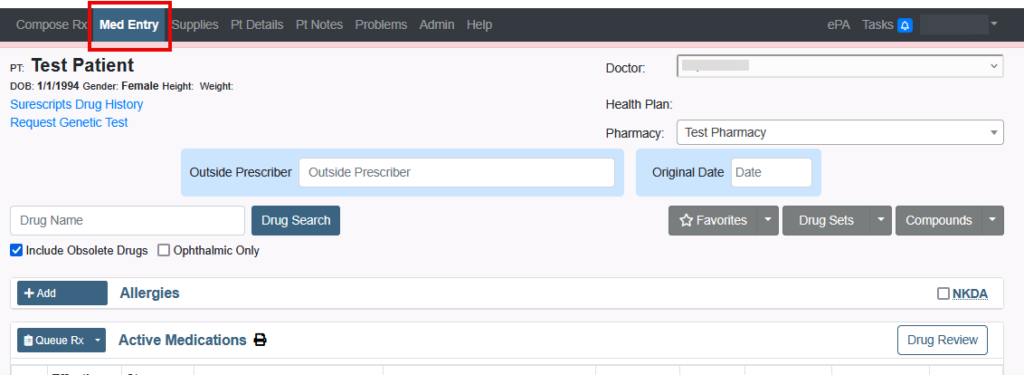
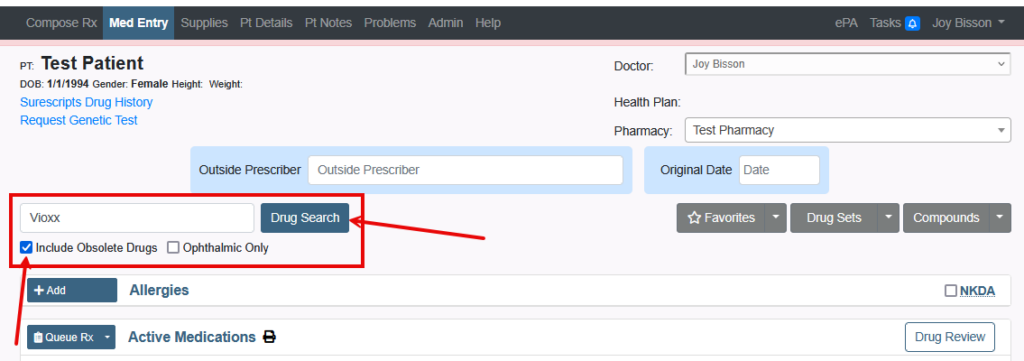
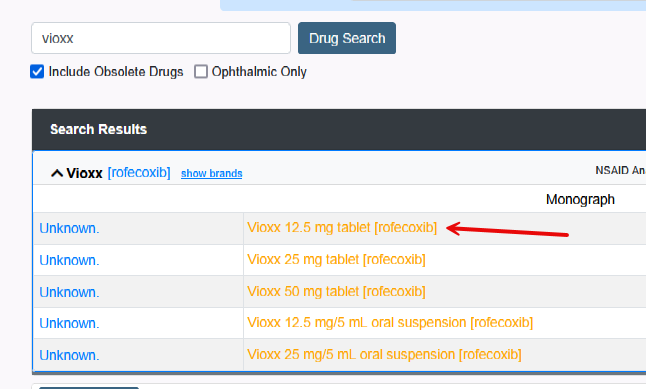
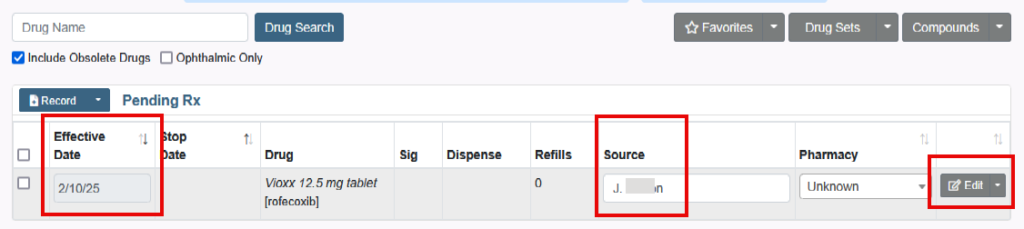
All medications have a National Drug Code (NDC). This article will define NDC and review the errors received when prescribing.
- What is a National Drug Code (NDC)
- What National Drug Code (NDC) error means when prescribing
- Why is a National Drug Code (NDC) not available
- How to Prescribe an Obsolete Drug or Medication without an NDC
- Document or Record Patient Taking an Obsolete Drug or No NDC
What is a National Drug Code (NDC)
All medications are assigned a National Drug Code (NDC) by the FDA when a new drug is released.
In its full form, it specifies brand, ingredient, manufacturer, and packaging. Surescripts, the electronic pharmacy network, requires than an NDC be sent with the transmitted prescription. In the past, when the doctor selected a medication without an NDC, NewCrop sent a “supply flag.” This eliminated the need for an NDC, allowing the prescription to slip through the transmission process. Surescripts no longer allows this workaround. Thus, the new feature you are seeing.
What National Drug Code (NDC) error means when prescribing
When a National Drug Code (NDC) is no longer available you will see the following error message, highlighted below:
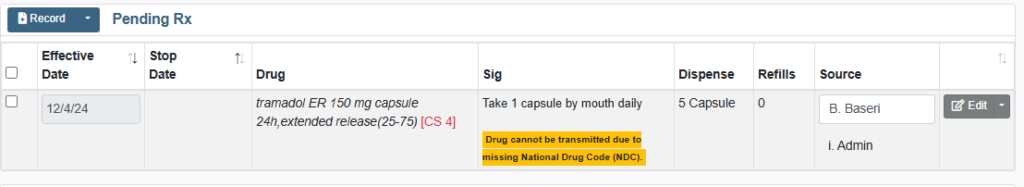
*Drug cannot be transmitted due to missing National Drug Code (NDC).*
1. Search for and select the “Generic” name of the medication.
2. Search for and select an “Alternative” medication.
Why is a National Drug Code (NDC) not available
There are at least two cases when a National Drug Code (NDC) is not available:
1. “Ingredient” when there is only a branded drug.
- Many doctors, especially academics, always prescribe by the ingredient. The pharmacy has no problem filling the rx. However, Surescripts no longer allows this. Therefore, if the doc selects the ingredient, we force a switch to the brand.
- Note “ingredient” not “generic”. Generic means the patent is expired and there are often multiple manufacturers. This drug does not have a “generic.” It does have the ingredient and the single brand name.
2. “Obsolete” brand name.
- This occurs when an older drug is no longer manufactured under the brand name, but the ingredient is available as a generic.
- An example is “Amoxil”, an old brand name for “Amoxicillin”. Doctors still hand-write “Amoxil”. The pharmacy has no problem dispensing generic “Amoxicillin”.
- Surescripts will NOT allow you to transmit the Rx of “Amoxil.”
- If an obsolete brand name is selected, the user will be forced to switch to the equivalent generic.
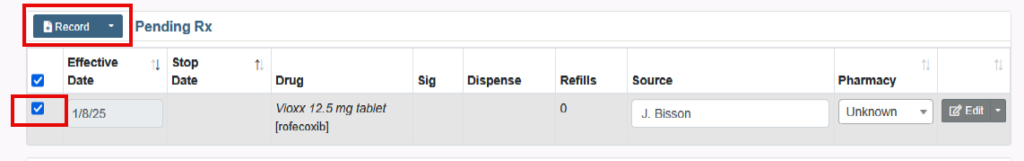
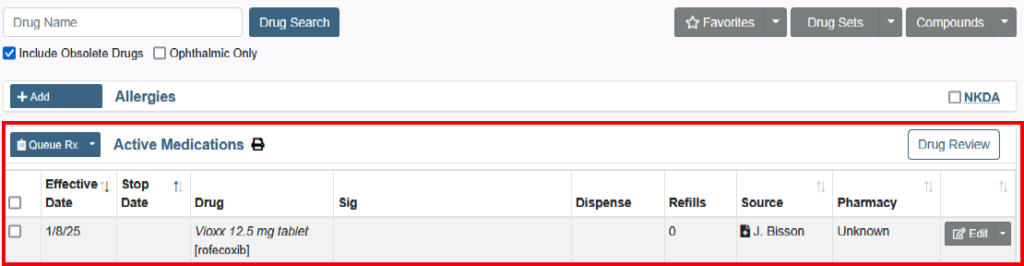
How to Prescribe an Obsolete Drug or Medication without an NDC
You will not be able to prescribe medications that are obsolete or marked as having no NDC.
But there are the following options:
1. Search for Generic Brand of the medication.
- Next to the Name Brand, in brackets, is the Generic Name of the medication.
- Clicking the Generic Brand will take you to the available forms of the Generic Brand medication.
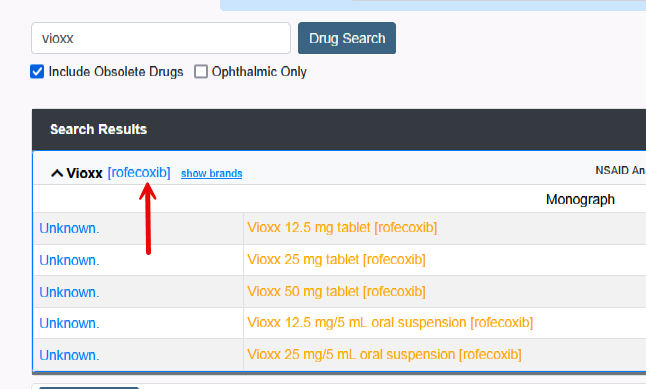
2. Search for Alternative Drug of the medication.
- Click Show Brands to see a list of medications.
- Click Monograph to view LexiComp for possible alternatives.
- Call the Pharmacy to ask for alternative medications.